Abstract
Introduction: NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) for Chronic Myeloid Leukemia recommend taking certain comorbidities into consideration when selecting a 2-G TKI. Based on toxicity profiles, dasatinib or bosutinib is preferred for patients (pts) with heart disease, arrhythmias, pancreatitis, and/or hyperglycemia; nilotinib or bosutinib is preferred for pts with a history of lung disease and/or who are at risk for pleural effusion. The prevalence of these relevant comorbidities in pts with CML was reported to be high in the US managed care setting, particularly in pts aged ≥ 65 years (Jabbour et al. CLML 2015), supporting the NCCN Guidelines® recommendations. To better understand the patterns of 2-G TKI selection in the first-line setting, this study was designed to assess the prevalence of relevant comorbid conditions in pts newly diagnosed with CML and treated with dasatinib or nilotinib using US real-world databases.
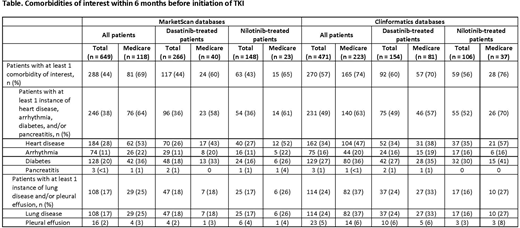
Methods: Data for pts diagnosed with CML between 4/1/2013 and 3/31/2016 were extracted from the Truven Health Analytics Commercial and Medicare MarketScan Research databases, and data for pts diagnosed with CML between 4/1/2013 and 12/31/2016 were extracted from the Clinformatics Commercial and Medicare Claims databases. Eligible pts were adults treated with a TKI (imatinib, dasatinib, nilotinib) within 6 months after initial CML diagnosis who had continuous enrollment data for 6 months prior to CML diagnosis and 12 months after TKI initiation. The first TKI prescription was defined as the index date. Comorbidities of interest as stated in the NCCN Guidelines® (heart disease, arrhythmia, diabetes, pancreatitis, lung disease, and pleural effusion) were assessed within 6 months before initiation of a TKI.
Results: A total of 649 pts and 471 pts were identified from the MarketScan and Clinformatics databases, respectively. Within these totals, 118 pts were identified from the MarketScan Medicare database, and 223 pts were identified from the Clinformatics Medicare database. The median age of pts from MarketScan was 55 years (dasatinib and nilotinib: 54 years), with 83% aged < 65 years (dasatinib: 86%; nilotinib: 85%). The median age of pts from Clinformatics was 63 years (dasatinib: 64 years; nilotinib: 62 years), with 54% aged < 65 years (dasatinib: 53%; nilotinib: 62%). Men comprised 57% (dasatinib: 58%; nilotinib: 55%) and 55% (dasatinib: 51%; nilotinib: 59%) of the population from MarketScan and Clinformatics, respectively. Forty-four percent of pts from MarketScan and 57% of pts from Clinformatics had at least 1 comorbidity of interest, as classified by the National Comprehensive Cancer Network® (NCCN®) (Table). For pts identified from the Medicare databases (typically aged > 65 years), 69% from MarketScan and 74% from Clinformatics had at least 1 comorbidity of interest. In the overall MarketScan and Clinformatics databases, respectively, 18% and 24% of pts with at least 1 instance of lung disease and/or pleural effusion were prescribed dasatinib, and 36% and 52% of pts with at least 1 instance of heart disease, arrhythmia, diabetes, and/or pancreatitis were prescribed nilotinib. In the MarketScan and Clinformatics Medicare databases, respectively, 18% and 33% of pts with at least 1 instance of lung disease and/or pleural effusion were prescribed dasatinib, and 61% and 70% of pts with at least 1 instance of heart disease, arrhythmia, diabetes, and/or pancreatitis were prescribed nilotinib. A relatively high proportion of pts treated with nilotinib had relevant comorbidities present at the time of treatment choice.
Conclusions: In this large retrospective analysis using 2 US claims databases, up to 57% of pts had comorbidities relevant to the treatment of CML with TKIs. Despite this high incidence of comorbidities and the recommendations in the NCCN Guidelines®, a significant proportion of pts were prescribed a 2-G TKI with a side-effect profile that could exacerbate preexisting comorbid conditions. These data suggest that physicians may not be consistently considering comorbidities when choosing a first-line 2-G TKI for the treatment of pts with CML in the real-world setting. This study did not review the impact of dose adjustments due to relevant comorbidities per the NCCN Guidelines®. To ensure optimal care of pts with CML, increased awareness of the importance of comorbidities when selecting a TKI is needed.
Jabbour:Novartis: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding. You:Bristol-Myers Squibb: Employment. Le:Bristol-Myers Squibb: Employment. Brokars:Bristol-Myers Squibb: Employment. Brun:Bristol-Myers Squibb: Employment. Makenbaeva:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal